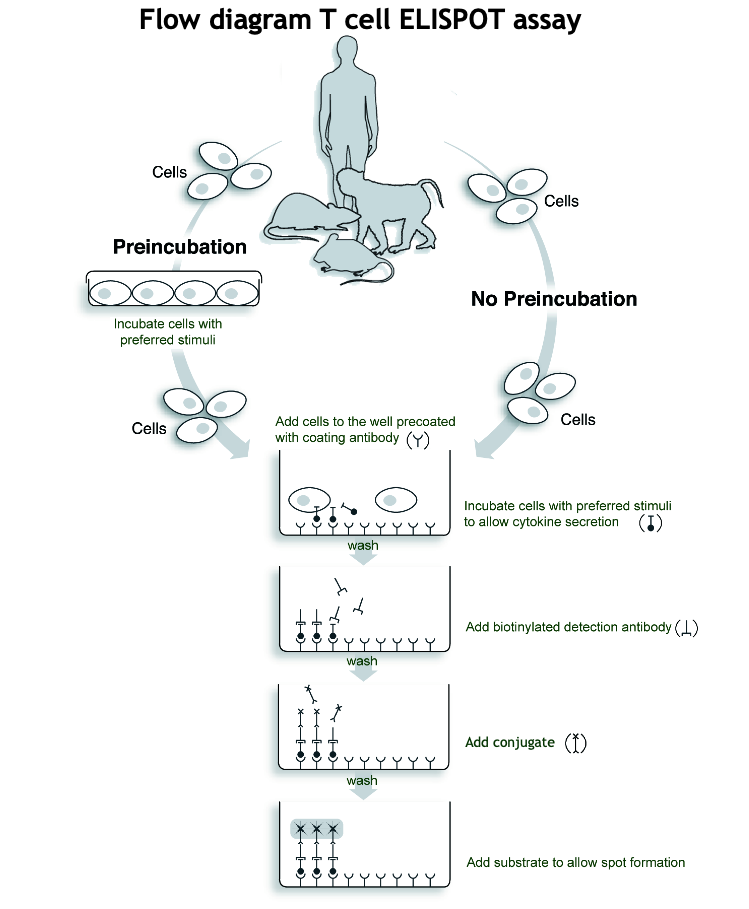
T cell ELISPOT assay
The antibodies in the monkey kits kits have been validated for detecting cytokine producing cells of various Old World monkeys including rhesus macaques, pig-tailed macaques, cynomolgous monkeys and baboons. Our ELISPOT kits for New World monkey have been validated for the detection of cytokines from marmoset.
Additionally, human and monkey "ELISPOT" kits have been developed for the detection of two cytokines released by a single T cell using fluorescent-labeled antibodies (the so-called FluoroSpot).
Intended use of the T cell ELISPOT assay
The ELISPOT assay is one of the most sensitive assays to monitor ex vivo cellular immune responses at the single cell level. The assay can accurately detect secreted proteins, such as cytokines, released by T cells in response to an antigen. The cell suspensions used in the test can be from blood (PBMC), lymphoid, spleen, bone marrow or CNS tissue.
Classical T cell monitoring assays (e.g. mixed lymphocyte reaction [MLR] and Cytotoxic T lymphocyte [CTL] assays) measure CD4+ or CD8+ T cell mediated immune responses. Both MLR and CTL assays have their drawbacks including the use of radioactivity, low throughput screening, reduced sensitivity in cryopreserved specimens and technical burden. RT-PCR analysis, to measure T cell responses can also be used. However, this assay detects mRNA rather than secreted protein.
The ELISPOT assay, which does not suffer from these shortcomings, has demonstrated to be more sensitive than an ELISA or intracellular cytokine staining.1-3 The high sensitivity is due to plate-bound antibodies that directly capture the secreted proteins released by the cell before they dilute in the cell culture medium, interact with cell-surface receptors on cells or are degraded by proteases. This characteristic allows the detection of very low frequencies of cytokine secreting cells (1/100,000) and also offers the possibility of high throughput screening.
Application
-
The ELISPOT assay has proven to be a sensitive and unique system for monitoring disease progression in human individuals or animals. Several studies have demonstrated that changes in the frequency of cytokine producing cells in different compartments of the body adequately reflect changes in immune function.4
-
The ELISPOT assay may be useful in understanding the development of disease in cases of new viruses cause disease and in early vaccine development studies, such as with the SARS-CoV-2 virus in the COVID-19 pandemic.5,6
-
The ELISPOT assay can be used to analyze T cell responses to predict allograft rejection.7
-
The ELISPOT assay can be used to determine the frequency of antigen-specific T cell responses in PBMC of vaccinated non-human primates8,9 and spleen cells of immunized rats10 and mice11.
-
The ELISPOT assay may be useful to better understanding how pathogens evade protective immunity.12
-
The ELISPOT assay has been used in the course of vaccination trials aimed at the induction of tumor-specific T cells.13,14
Brief description of the T cell ELISPOT procedure
Cells are incubated for a specific period of time in the wells of the ELISPOT plate coated with a high-affinity monoclonal antibody to which the cytokine, produced during incubation, binds. Subsequently, cells are washed away. Areas in which the cytokine is captured by the coated antibody are detected with a combination of biotinylated anti-cytokine detection antibodies and enzyme-labeled streptavidin. The final step in the assay is the addition of a substrate yielding a colored zone ('spot') that reveals the site of cytokine secretion.
The different steps of the T cell ELISPOT procedure are illustrated in the T cell ELISPOT assay Flow diagram.
Example of the Human T cell ELISPOT assay:
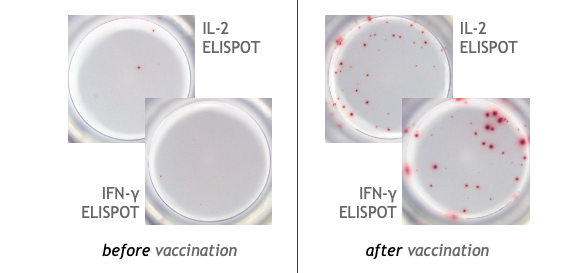
This is an example of IFN-γ and IL-2 specific spots produced by T cells in human PBMC.
The individual was intramuscularly vaccinated (booster vaccination) with a COVID-19 mRNA vaccine. PBMC from before and after vaccination were stimulated with a SARS-CoV-2 peptide pool and incubated for 24 hours on the ELISPOT plate (2x105 cells/well).
U-CyTech ELISPOT products
Follow these links to go to directly to our:
- Human ELISPOT products
- Monkey ELISPOT products
- Marmoset ELISPOT products
- Mouse ELISPOT products
- Rat ELISPOT product
References
- Tanguay S and Killion JJ (1994). Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 13: 259-63.
- Carter LL and Swain SL (1997). Single cell analyses of cytokine production. Curr. Opin Immunol. 9: 177-82.
- Herold KC et al. (2009). Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes. 58: 2588-95.
- Van der Meide PH et al. (1998). Discontinuation of treatment with IFN-beta leads to exacerbation of experimental autoimmune encephalomyelitis in Lewis rats. Rapid reversal of the antiproliferative activity of IFN-beta and excessive expansion of autoreactive T cells as disease promoting mechanisms. J. Neuroimmunol. 84:14-23.
- U-CyTech products used in this study:
- Zhao B et al. (2021). Alterations in Phenotypes and Responses of T Cells Within 6 Months of Recovery from COVID-19: A Cohort Study. Virol. Sin. Oct;36(5): 859-868
- U-CyTech products used in this study:
- Feng L et al. (2020). An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun. 11: 4207.
- U-CyTech products used in this study:
- Monkey species: Macaca mulatta
- Mendoza Rojas A et al. (2023). Alloreactive T cells to Assess Acute Rejection Risk in Kidney Transplant Recipients. Transplant Direct. 9(5): e1478.
- U-CyTech products used in this study:
- Rollier CS et al. (2016). T- and B-cell responses to multivalent prime-boost DNA and viral vectored vaccine combinations against hepatitis C virus in non-human primates. Gene Ther. 23: 753-759.
- U-CyTech products used in this study:
- Monkey species: Macaca mulatta
- Karmakar S et al. (2014). Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine 32: 1296-303.
- U-CyTech products used in this study:
- Monkey species: Papio anubis
- Liu GX et al. (2009). Mucosal and systemic immunization with targeted fusion anti-caries DNA plasmid in young rats. Vaccine 27: 2940-7.
- U-CyTech products used in this study:
- Liu X et al. (2023). A mosaic influenza virus-like particles vaccine provides broad humoral and cellular immune responses against influenza A viruses. NPJ vaccines. 8(1): 132.
- U-CyTech products used in this study:
- Li Z et al. (2022). Impaired T lymphocyte responses during childhood Staphylococcus aureus infection. J. Infect. Dis. 225: 177-85.
- U-CyTech products used in this study:
- Mirandola L et al. (2019). A novel method for efficient generation of antigen-specific effector T-cells using dendritic cells transduced with recombinant adeno-associated virus and p38 kinase blockade. J. Transliterates. Med. 17: 424.
- U-CyTech products used in this study:
- Peng S et al. (2022) Combination neoantigen-based dendritic cell vaccination and adoptive T-cell transfer induces antitumor responses against recurrence of hepatocellular carcinoma. Cancer Immunol. Res. 10(6): 728-744.
- U-CyTech products used in this study: