Troubleshooting B cell ELISPOT assay
What seems to be the problem?
- High background
- Confluent spots or poorly defined spots
- Faintly stained or no spots in positive controls
- Low spot frequency
- Small spots
- Large spots
- Poor consistency of replicates
- Blank areas
- High spot frequency in negative controls
My question is not mentioned here, how can I contact U-CyTech?
Notes:
RT: room temperature; temperature between 20 °C and 26 °C
To abbreviations
| Examples | |
|
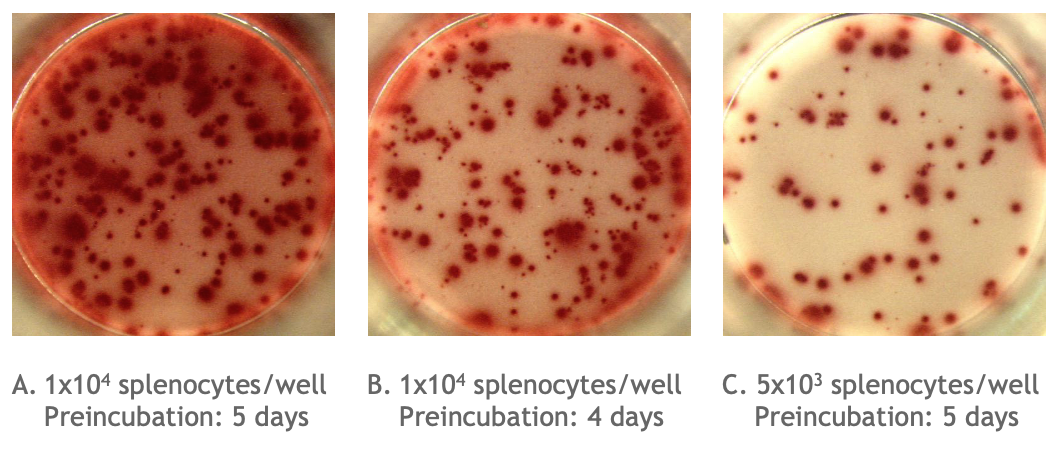
Example of the Monkey IgG B cell ELISPOT assay:
Total number of IgG spots producing memory B cells in spleen cells of cynomolgus monkey (Assay I). Memory B cells were activated in vitro (2x106 cells/ml) in the presence of recombinant IL-2 and R848 as stimuli. Thereafter, the cells were transferred to the ELISPOT plate and were incubated for 24 hours. |
|
|
Picture A is an example of high background / poorly defined spots in an ELISPOT well. The high background / poorly defined spots due to too many spots is caused either by:
|
|
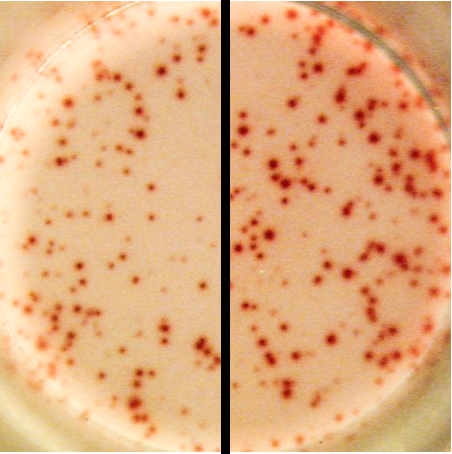 |
An example of faintly stained spots (left) compared to correctly stained spots (right) in an ELISPOT well. Faintly stained spots are caused either by an improper AEC substrate solution, improper handling of AEC stock solution or a poor color development. |
I am experiencing a high background in the ELISPOT wells. What could have happened?
High background can be due to a high background staining or to too many spots. There could be various reasons:
- Inadequate washing: follow the “Directions for washing” carefully.
- Nonspecific binding: Do not use human, non-human primate or rodent serum as growth supplement. The antibodies in the serum will interfere with spot formation. Other serum used in the culture medium should be selected on low background staining.
- Contaminated working solutions or cell culture: solutions should not be used when they have become turbid or an indication of bacterial or fungal growth. Use clean containers/pipet tips for the transfer of solutions into the wells of the ELISPOT plate.
- Overdeveloped plate: reduce the incubation time of color development.
- Carryover of secreted proteins (i.e. antibodies) produced during preincubation step: ensure effective washing of collected cells after preincubation and before adding cells to the ELISPOT plate. See: "Cell sample preparation".
- Incomplete drying of PVDF membranes after completion of the ELISPOT assay: allow the PVDF membranes to dry completely (protected from light at RT) prior to spot counting.
- Too many cells in the ELISPOT well: decrease the cell concentration in the wells of the ELISPOT plate by making some dilutions that will result in formation of distinct spots (optimally 50-250 spots/well).
- Improper incubation period of cells in the ELISPOT plate: decrease incubation time of cells in the ELISPOT plate or during the preincubation step.
The spots I see are poorly to define or confluent. What went wrong?
There could be several reasons for confluent or poorly defined spots:
- Too many cells in the ELISPOT well: decrease the cell concentration in the wells of the ELISPOT plate by making some dilutions that will result in formation of distinct spots (optimally 50-250 spots/well).
- Improper incubation period of cells in the ELISPOT plate: decrease incubation time of cells in the ELISPOT plate or during the preincubation step.
- Moving ELISPOT plate during cell incubation: prevent the plate from being moved during the cell incubation step. Even minor vibrations (e.g. closing the door of the incubator) can affect spot formation.
- Dust particles: prior to spot counting, remove dust particles by blowing compressed air (4-5 bar) into the wells.
The spots I see are faint or I find no spots at all in my positive controls. What went wrong?
Finding no spots at all in the positive controls suggest a problem with the cells or the assay did not work properly. Faintly stained spots can be due to a problem that occurs during the assay procedure. Several causes could be the reason of no spots or faintly stained spots:
- Reduced viability of cells: check the viability of the cells before using in the ELISPOT procedure, especially when using cryopreserved and thawed cells. Ensure the period between blood draw and PBMC or spleen cells isolation is as short as possible (preferably less than 8 h). See directions for “Cell collection and handling”.
- Use of PBS tablets for preparing coating antibody solution: the filler in tablets interferes with the coating process. Use sterile liquid ready-to-use PBS instead. Thermo Fisher Scientific cat. no. 10010 is recommended.
- Degradation of antibodies: follow recommended storage conditions.
- Degradation of streptavidin-HRP conjugate: follow recommended storage conditions. Ensure protection from light and avoid prolonged storage at ≥4 °C.
- Improper handling of AEC stock solution: follow recommended storage conditions. Ensure protection from light and avoid prolonged storage at ≥0 °C. AEC solution must not come into contact with polystyrene plastics.
- Improper antibody or conjugate solution: ensure appropriate dilution of antibodies and conjugate.
- Improper AEC solution: ensure accurate dilution of AEC solution. Always use freshly prepared AEC solution and keep it protected from light. AEC solution must not come into contact with polystyrene plastics.
- Incorrect incubation times or temperature: ensure correct incubation times and temperature of the ELISPOT reagents. Reagent solutions should be at RT before use. Check function of the CO2 incubator (37 °C, 100% humidity, 5% CO2). Optimal cell incubation times should be determined experimentally. Test different (pre)incubation times. Check "Cell sample preparation".
- At some point no reagent was added: check the liquid level of all wells after adding new reagent.
- Poor color development: increase the incubation time of color development. Ensure the AEC solution is incubated while protected from light.
- Bleaching of spots: store ELISPOT plates at a dry place at RT protected from light. Enzymatic stained spots may bleach.
- Omitting prewetting step: it is important to prewet the hydrophobic PVDF membrane with ethanol to assure optimal binding of the coating antibodies to surface.
- Drying out of the PVDF membrane: do not allow the PVDF membrane to dry during the procedure. If this occurs directly after prewetting, repeat prewetting step.
- Insufficient binding of the antigen of interest to the PVDF membrane: use a different antigen. Alternatively, use the provided coating antibody in combination with a biotinylated antigen of interest as detection instead of the provided detection antibody. This procedure prevents the search for a PVDF membrane-binding antigen, and reduces the amount of antigen required.
I am getting a low frequency of spots. What could have happened?
There could be several reasons for low spot frequency:
- Clumping of cells: resuspend cells gently but thoroughly, to gain a homogeneous cell suspension, before the cells are transferred into the wells of the ELISPOT plate.
- Reduced viability of cells: check the viability of the cells before using them in the ELISPOT procedure, especially when using cryopreserved and thawed cells. Ensure the period between blood draw and PBMC or spleen cells isolation is as short as possible (preferably less than 8 h). See directions for “Cell collection and handling”.
- Cell concentration in ELISPOT wells is too low: increase the cell concentration in the wells of the ELISPOT plate. Make several dilutions of cells to find the optimal dilution of cells resulting in formation of distinct spots (optimal: 50-250 spots/well). Do not add more than 3x105 cells/well, because cells wil cause multiple cell layers, resulting in poor spot formation.
- Insufficient numbers of in vivo activated B cells in the blood or spleen are present: collect cells at a different time point after in vivo exposure or activated memory B cells in vitro by a preincubation step. See "Cell sample preparation".
- Inadequate incubation time of the cells: determine the optimal (pre)incubation time of the cells by increasing or decreasing the (pre)incubation time.
- The underdrain of the ELISPOT plate has not been removed: remove and discard underdrain after the detection antibody incubation step and wash both sides of the PVDF membrane in each wash step thereafter.
The spots I see are all small. What went wrong?
There is one mean reason for small spots:
- Inadequate incubation time of cells: prolong (pre)incubation time of the cells on ELISPOT plate.
The spots I see are all large. What went wrong?
There is one mean reason for large spots:
- Inadequate incubation time of cells: shorten incubation time of the cells on ELISPOT plate.
I am experiencing a poor consistency of replicates between the ELISPOT wells. What could have happened?
There are several reasons for a poor consistency of replicates:
- Inaccurate pipetting: ensure accurate pipetting volume. Check pipettes.
- Clumping of cells: resuspend cells gently but thoroughly, to gain a homogeneous cell suspension, before the cells are transferred into the wells of the ELISPOT plate.
- Evaporation of solutions: ensure adequate humidity. Check function of the CO2 incubator (37 °C, 100% humidity, 5% CO2). Ensure accurate sealing of the plate.
- Inaccurate temperature distribution during incubation steps: do not stack the plates during incubation.
- Inadequate washing: follow the “Directions for washing” carefully.
- At some point no reagent was added: check the liquid level of all wells after adding new reagent.
I see blank areas in between the spots. What went wrong?
There are various reasons for blank areas:
- Cells are unevenly distributed: resuspend cells gently but thoroughly, to gain a homogeneous cell suspension, before the cells are transferred into the wells of the ELISPOT plate. Do not add any solution once the cells are in the wells of the ELISPOT plate.
- Damage of the coating layer: ensure not to scratch the bottom of the well with a pipet tip.
- Inaccurate prewetting of the PVDF membrane: do not allow the PVDF membrane to dry during the procedure. If this occurs directly after prewetting, repeat prewetting step.
- Foam forming during washing: the spout of the squirt bottle may be too narrow and should be enlarged.
- The underdrain of the ELISPOT plate has not been removed: remove and discard underdrain after the detection antibody incubation step and wash both sides of the PVDF membrane in each wash step thereafter.
I am experiencing a high frequency of spots in the negative controls. What could have happened?
Finding a high number of spots (i.e. ≥ 30 spots/well when working with 2x105 cells/well) in the negative controls suggest that either the cells spontaneously secrete antibodies or the assay did not work properly. Several reasons could explain a high frequency of spots in the negative controls:
- Recently in vivo activated B cells will spontaneously secrete antibodies and the immune system of the donor can still be active without us realizing it. Therefore, even when cells are not preincubated with stimuli, the cells spontaneously can secrete antibodies.
- False positive results due to reagents or cell culture media: reagents and cell culture media ingredients, like serum, should not trigger cells. Do not use human, non-human primate or rodent serum as growth supplement. The antibodies in the serum will interfere with spot formation. Serum or new medium (ingredients) should be selected on low background staining. Check whether the background control (no coating added but all other reagents and cells) has no spots. When this is not the case, one or more of the used reagents is causing false positive results.
- Inaccurate cell collection and handling. See: "Cell collection and handling" for our guidelines.
My question is not mentioned here, how can I contact U-CyTech?
The researchers of U-CyTech build on >20 years of experience on the development and performance of the ELISPOT assays for human, monkey and mouse species. They are more than happy to help you with additional advice and to share their experiences.
Please contract our customer service:
E-mail: cs @ ucytech.com
(back to top or to B cell ELISPOT assay guidelines)
Note: