Interleukin 8 (CXCL8)
Interleukin 8 (IL-8), a small soluble protein belonging to the CXC chemokine family, is a chemotactic factor that attracts neutrophils, basophils and T cells to an inflammatory site. It specifically regulates neutrophil adhesion, migration and respiratory burst activity, but also has immunomodulatory activities, such as the induction of matrix metalloproteinase-9 (MMP-9) expression and the release of TNF-related apoptosis-inducing ligand (TRAIL).
Several other names for IL-8 circulate in literature, including CXCL8, T cell chemotactic factor, neutrophil-activity peptide 1, and Beta-thromboglobulin-like protein. Although the designation CXCL8 is officially endorsed by the 'Chemokine Nomenclature Subcommittee of the International Union of Immunological Societies', the name IL-8 is still most commonly used.
All cells with toll-like receptors can secrete IL-8, and the protein can exist in several active isoforms. There are two most abundant is-forms: a 72 amino acid isoform mainly releaesed by macrophages in culture, and a 77 amino acid variant secreted by non-immune cells. 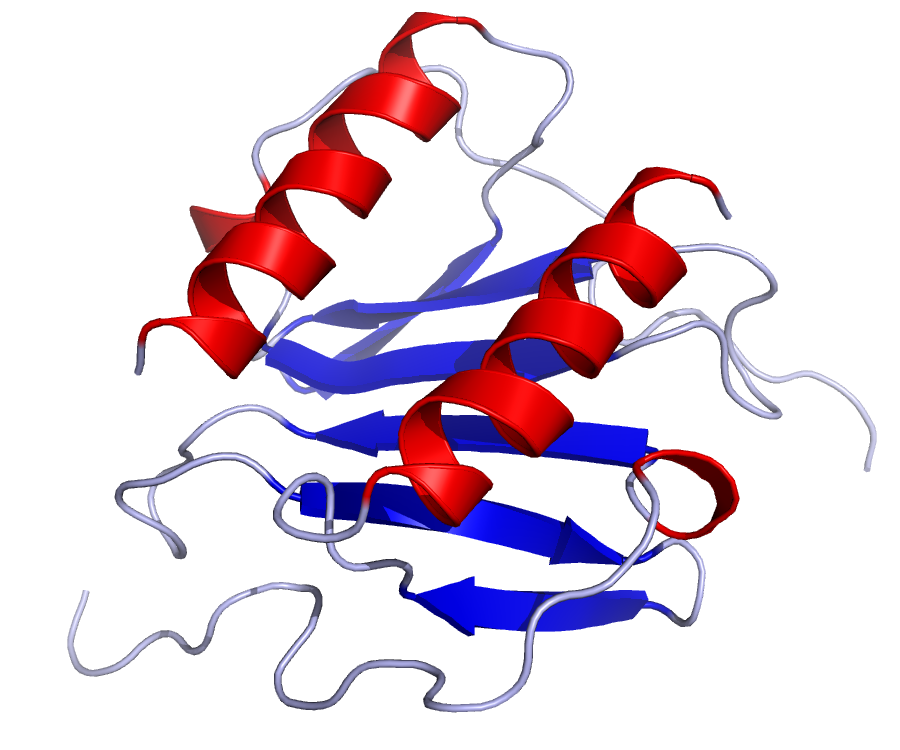

Figure This is a structure of IL-8 created using the data from Protein Data Bank (PDB: 1IL8) and rendered using PyMOL.
The biological effects of IL-8 are mediated via two Guanine-protein-coupled receptors (GPCRs): CXCR1 (IL-8RA) and CXCR2 (IL-8RB). CXCR1 is activated by IL-8 and by granulocyte chemotactic protein-2 (GCP-2/CXCL6). CXCR2 is more promiscuous because it can be activated not only by IL-8, but also by many other CXC chemokines including CXCL1, CXCL2, CXCL3, CXCL5, GCP-2/CXCL6, and CXCL7.
In normal tissue, the level of IL-8 is low or not detectable, but is exponentially increased (up to 100-fold) in response to pro-inflammatory cytokines such as TNF, IL-1, bacterial or viral virulence factors and oxidative stress.