B cell ELISPOT assay
U-CyTech biosciences offers B cell ELISPOT kits for detection of human, monkey and mouse antibody secreting cells (ASCs) in blood or tissue samples.
Long-lived memory B cells and plasma cells are responsible for the humoral immunity elicited by most vaccines or infections. The traditional method to monitor a humoral response after immunization or infection is to quantify specific antibody levels in serum by ELISA. However, the actual correlate of protection is mediated by circulating antibodies in combination with antibody producing memory B cells. The B cell ELISPOT assay has proven particularly useful to determine the number of individual antibody-producing memory B cells in single cell suspensions e.g. peripheral blood, lymph nodes or spleen cells.
Memory B cells, which have a long lifespan, play a central role in the rapid response after re-exposure to the specific antigen. While being quiescence, the presence of memory B cells cannot be determined by ELISA. A major advantage of the B cell ELISPOT assay is its ability to activate ex vivo antigen-specific memory B cells, whereafter they can be measured.
The B cell ELISPOT is the assay of choice to determine the degree and level of (vaccine-induced) protection against a given infection. The B cell ELISPOT assay is useful in several areas of biomedical research including vaccine development1,2, infection research (i.e. herpes zoster, COVID-19 and hepatitis B)3,4,5,6,7, drug treatment8, autoimmune diseases and allergy9.
Activation of memory B cells
In general, ASCs are short-lived, while memory B cells have a long lifespan and play a critical role in long-term immunity against a given antigen. Because of low frequencies in peripheral blood and secondary lymphoid tissues, and lack of antibody secretion, memory B cells need first to be activated and expanded in vitro by a 2-5 days polyclonal stimulation before they can be determined by the B cell ELISPOT assay.
B cell ELISPOT procedure
Our B cell ELISPOT assay has a high sensitivity and easy performance, making the assay ideally suited for monitoring memory B cell responses at the single cell level.
A cell suspension of activated B cells (in vitro activated memory B cells or ASC present in vivo) is transferred into the wells of the ELISPOT plate coated with either:
- Assay I: antibodies directed against a specific class of immunoglobulin (e.g. IgG)
- Assay II: antigen of interest*
Antibodies released by the activated B cells are captured by the coated antibody (Assay I) or antigen (Assay II). Subsequently, cells are washed away. Areas where secreted antibody is bound, are detected with a combination of biotinylated anti-immunoglobulin detection antibodies and enzyme-labeled streptavidin. The final step in the assay is the addition of a substrate yielding a colored zone ('spot') showing the site of antibody secretion, representing either the total number of ASC (Assay I) or antigen-specific ASC (Assay II).
The protocols Assay I and Assay II, determining the total number of ASCs and antigen-specific ASCs, respectively, are usually performed in parallel. Since the in vitro stimulation step of memory B cells expands the cells, the number of antigen-specific memory B cells is usually compared to the total number of immunoglobulin ASCs to obtain an accurate estimate of the frequency of antigen-specific ASCs present.
The different steps of the B cell ELISPOT procedure are illustrated in the B cell ELISPOT assay Flow diagram.
*) Alternatively, antigen-specific immunoglobulin secreting B cells can also be analyzed by using antibodies directed to a specific class of immunoglobulin (e.g. IgG) as coating and a biotinylated antigen of interest as detection. This reduces the amount of specific antigen required.
Example of the Human IgG B cell ELISPOT assay:
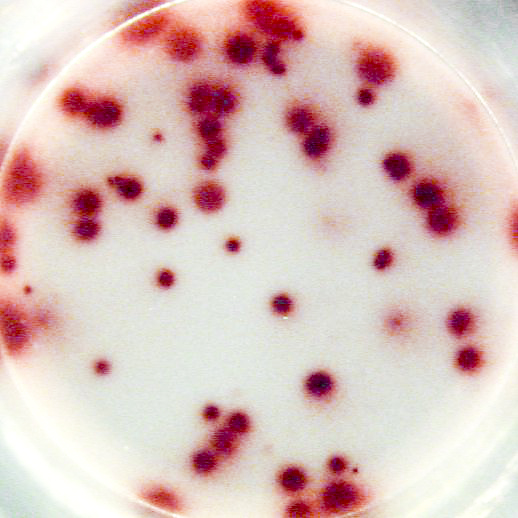
Example of IgG specific spots produced by memory B cells in human PBMC.
The individual received a booster vaccination with tetanus toxoid more than 5 years ago.
Memory B cells were activated in vitro by a preincubation (2x106 cells/ml) of 5 days in the presence of recombinant IL-2 and R848 as stimuli. Thereafter, the cells were transferred to the ELISPOT plate and were incubated for 20 hours.
Left: Total number of IgG producing cells (2x103 cells/well) - Assay I
Right: Tetanus toxoid specific IgG producing cells (1x105 cells/well) - Assay II
U-CyTech ELISPOT products
Follow these links to go to directly to our:
References
Click on the authors for the abstract of the below mentioned papers.
- Sun P et al. (2021). T cell and memory B cell responses in tetravalent DNA, tetravalent inactivated and tetravalent live-attenuated prime-boost dengue vaccines in rhesus macaques. Vaccine 39951): 7510-20.
- U-CyTech products used in this study:
- Monkey species: Macaca mulatta
- Chen X et al. (2024). Adjuvant activity of cordycepin, a natural derivative of adenosine from on an inactivated rabies vaccine in an animal model. Heliyon 10(2): e2412.
- U-CyTech products used in this study:
- Kho MML et al. (2022). Boosting the VZV-specific memory B and T cell response to prevent Herpes Zoster after kidney transplantation. Front Immunol. 13: 927734.
- U-CyTech products used in this study:
- Moore T et al. (2022). SARS-CoV-2-specific memory B cell responses are maintained after recovery from natural infection and postvaccination. Viral Immunol. 35(6): 425-436.
- U-CyTech products used in this study:
- Van Besouw NM et al. (2016). Herpes zoster after lung transplantation boosts varicella zoster virus-specific adaptive immune responses. J Heart Lung Transplant. 35(12): 1435-42.
- U-CyTech products used in this study:
- Mori T et al. (2023). Toll-like receptor 7 agonist, GS-986, is an immune-stimulant inducing follicular helper T cells and expanding HBs antigen-specific B cells in vitro.Liver Int. 43(6): 1213-1224.
- U-CyTech products used in this study:
- He T et al. (2023). Decreased antibody response to influenza vaccine with an enhanced antibody response to subsequent SARS-CoV-2 vaccination in patients with chronic hepatitis B virus infection. Immune Inflamm Dis. 11(1): e759.
- U-CyTech products used in this study:
- Matino D et al. (2015). IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J Clin Invest. 125: 3766-81.
- U-CyTech products used in this study:
- Evans H et al. (2015). Ten weeks of infection with a tissue-invasive helminth protects against local immune complex-mediated inflammation, but not cutaneous type I hypersensitivity, in previously sensitized mice. J Immunol. 195(7): 2973-84.
- U-CyTech products used in this study: