For your convenience, we have collected the frequently asked questions (FAQs) on our B cell ELISPOT, T cell ELISPOT assay and FluoroSpot assay. Please find them below.
ELISPOT and FluoroSpot assay - general questions |
|
| What is an ELISPOT assay? | |
|
The enzyme-linked immunospot (ELISPOT) assay is a widely used method for monitoring immune responses. The assay is a highly sensitive method for the ex vivo quantification of cytokine or antibody secreting cells after stimulation with an appropriate stimulus in vitro (Cox et al. 2006). In general, each cell can be detected by ELISPOT if a characteristic protein is released and specific high affinity antibodies recognizing the protein are available. The ELISPOT assay is comparable to an enzyme-linked immunosorbent assay (ELISA) and is based on the same immunochemical 'sandwich' principle. The major difference is that an ELISPOT is a combination of both an immunoassay and bioassay because living cells are cultured directly in the wells of the ELISPOT plate. |
|
| What is an FluoroSpot assay? | |
|
The dual color FluoroSpot assay is a modification of the T cell ELISPOT assay. It is developed for simultaneous detection of two cytokines released by a single T cell. The assay is based on the use of fluorescent conjugates, which can be visualized by fluorescence microscopy or by an ELISPOT reader equipped with a fluorescent light source.
|
|
| In which area of research should an ELISPOT/FluoroSpot assay be used? | |
|
The ELISPOT/FluoroSpot assay can be used in many areas of research such as cancer, infectious disease, autoimmune disease, allergy and organ transplantation. The assay is particularly effective for the measurement of antigen-specific responses post-vaccination in peripheral blood cell preparations. For more information, please see ELISPOT applied in research. |
|
| What type of information provides an ELISPOT/FluoroSpot assay? | |
|
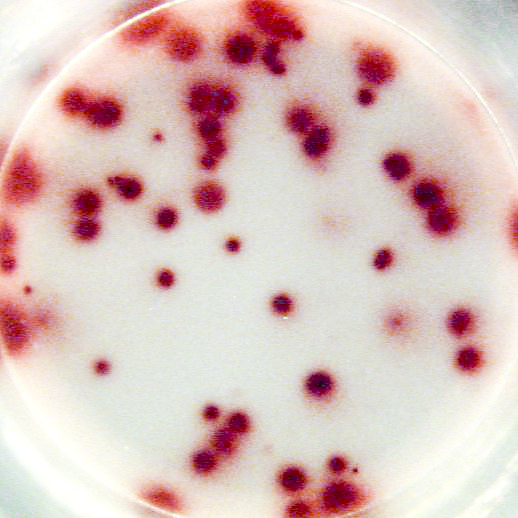
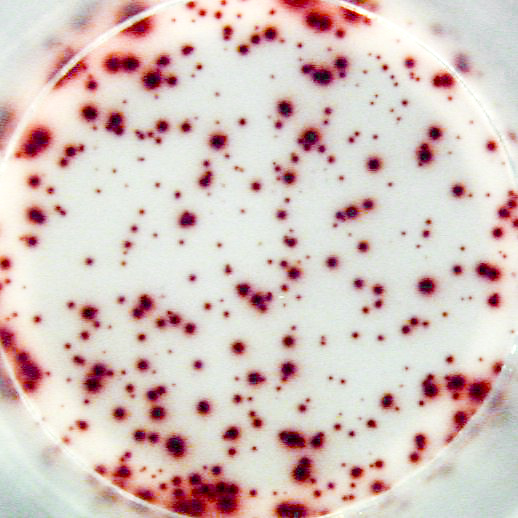
The most important information that can be achieved from ELISPOT/FluoroSpot assays is the frequency of antigen-specific T or B cells within a pool of different cell types. This frequency reflects the extent of the cellular of humoral immune response. Pictures:
|
|
ELISPOT and FluoroSpot assay - specific questionsWhich cell types can be analyzed by ELISPOT or FluoroSpot assay? In principle any type of cell that secrete proteins can be investigated at single cell level by the ELISPOT/FluoroSpot assay. Although, so far the technique has mainly been used to identify cytokine secretion by antigen-activated T cells and antibody secretion by B cells from peripheral blood or spleen cell preparations. Adherent cells can also be analyzed in the ELISPOT assay. However, when working with adherent cells, please keep in mind that a lysis step (see below) is necessary to remove all cells from the ELISPOT plate before proceeding with the detection step.
|
|
| Can I use all strains of inbred rodents and non-human primates in the T cell ELISPOT or FluoroSpot assay? | |
|
Some animal models are natural skewed to a Th1- or Th2-like response. In addition, some animals may only have a Th2 (or Th1) response if the animals have been stimulated with an appropriate antigen in vivo or if they are suffering from Th2-like (or Th1-like) disease. Therefore, please check if your animal model is expected to be able to produce the cytokine of interest.
|
|
| Are there special requirements and storage conditions for blood? | |
|
Whole blood should be kept at room temperature (RT; 20-26 °C) until processing. Do not refrigerate. If collected elsewhere, samples should be shipped at ambient temperature to the desired laboratory. Samples should not be at RT for more than 8 h after blood draw. For the T cell ELISPOT and FluoroSpot assay, blood samples can be gently mixed on a tube roller until PBMC isolated. The recommended anti-coagulants are heparin and citrate. For both substances there are no reported adverse effects on cellular function in the ELISPOT and FluoroSpot assay.
|
|
| How long could I preincubate the cells? | |
|
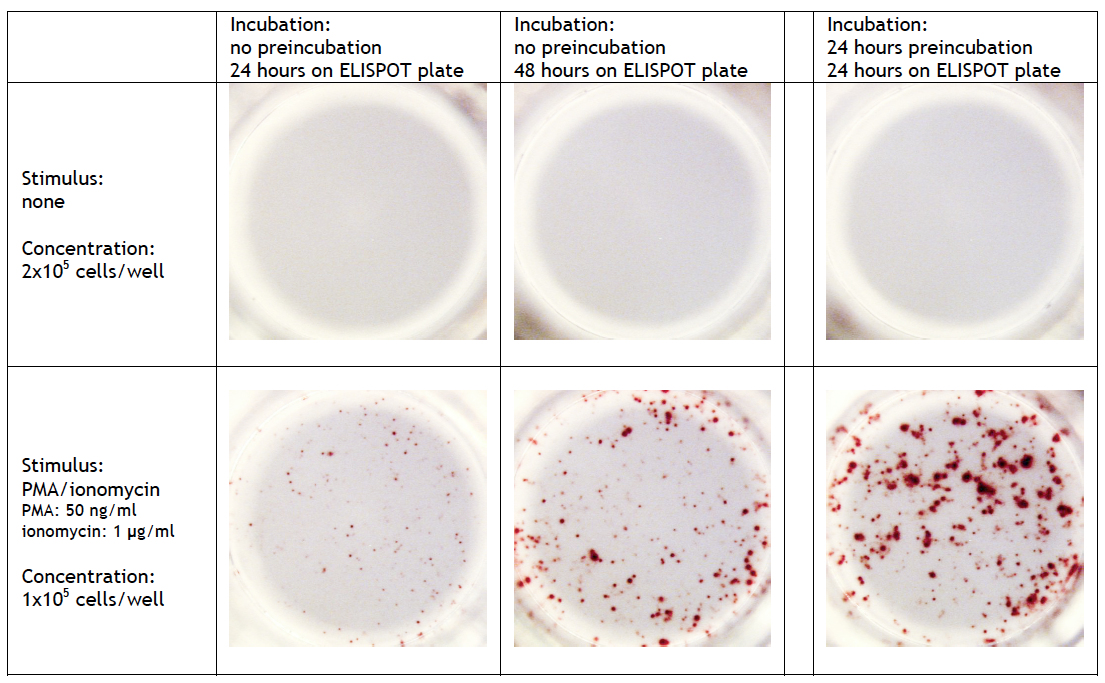
T cell ELISPOT assay: A 24-42 h preincubation step at high cell density (>106 cells/well/ml) can be required when full-length proteins or long peptides are used as stimulus in the ELISPOT assay. These antigens must first be internalized, processed and presented by antigen-presenting cells (APCs) via MHC class I/II molecules before they can stimulate cytokine release by T cells. The high number of cells enhances the probability of contact between stimulating and responding cells. Omitting this step may results to a significant lower frequency of spot forming cells. On the other hand, small (synthetic) peptides (8-12 mer) can directly be presented by APC to CD8+ cells and consequently need no preincubation step. A preincubation step can also be useful when the analyte of interest requires a longer incubation. Detailed information can be found at Cell sample preparation T cell ELISPOT assay. |
|
|
Example of IL-2 specific spots produced by mouse (C57bl/6) splenocytes using the Mouse IL-2 T cell ELISPOT kit (CT435-PR):
|
|
FluoroSpot assay: A 24-48 h preincubation step at high cell density (>106 cells/well/ml) is required for optimal results. Omitting this step leads to a significant lower frequency of spot forming cells and/or smal sized spots. |
|
|
B cell ELISPOT assay: Quiescent memory B cells do not produce antibodies in significant quantities in vivo. Therefore, a preincubation step of several days (2-5 days) in vitro at high cell density (>106 cells/well/ml) and with appropriate polyclonal stimuli is always required to activate these memory B cells. Detailed information can be found at Cell sample preparation B cel ELISPOT assay.
|
|
| How critical is the culture medium? | |
|
The recommended cell culture medium is RPMI-1640 supplemented with 2 mM L-Glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 10% fetal calf serum (FCS). It should be mentioned that some batches of FCS may non-specifically activate the cells and can therefore be the cause of background spot formation. Different batches of FCS should therefore be pretested on both low background reactivity as well as high antigen-specific responses before using it in the ELISPOT/FluoroSpot assay. In the T cell ELISPOT assay human, non-human primate or rodent serum can be used as alternative for FCS. This serum should selected on low background reactivity/high antigen-specific responses as well. When the T cell ELISPOT assay is performed without preincubation step, serum-free medium (AIM-V) supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin can also be a good alternative. Do not use human, non-human primate or rodent serum as growth supplement in the B cell ELISPOT assay. The antibodies in the serum will interfere with spot formation.
|
|
| What about ELISPOT and FluoroSpot assay controls? | |
|
Appropriate controls are important for the validation of the ELISPOT and FluoroSpot assay. Choice of controls depends on the cells of interest and type of experiment. Positive control in the T cell ELISPOT and FluoroSpot assay Both antigen-specific and polyclonal stimuli can be used. However, antigen-specific stimuli are preferred since cells of various species respond differently to polyclonal stimuli. For the human system, vaccine proteins (tetanus toxoid, hepatitis B, etc.) or a pool of synthetic peptides of common human viral epitopes are excellent positive controls. When using vaccine proteins, it should of course be known whether individuals have been vaccinated. Some animal models are skewed to a Th1- or Th2-like response. This can result in a low number of spots in the positive control. Therefore, please check if your animal model is expected to be able to produce the cytokine of interest. Negative control in the T cell ELISPOT and FluoroSpot assay Spontaneously secreting cells: if these cells are not triggered by e.g. culture medium ingredients (false positive results that should be avoided), they may be caused by an activated immune system of the donor without us realizing it. The number of spontaneously occurring spots may vary from donor to donor and at different time points for a donor within the observation period. Background control in the T cell ELISPOT and FluoroSpot assay
Positive control in the B cell ELISPOT assay (memory B cells only) Background control in the B cell ELISPOT assay To: An overview of general cell stimuli commonly used in ELISPOT and FluoroSpot assays
|
|
| What type of spot detection systems are available? | |
|
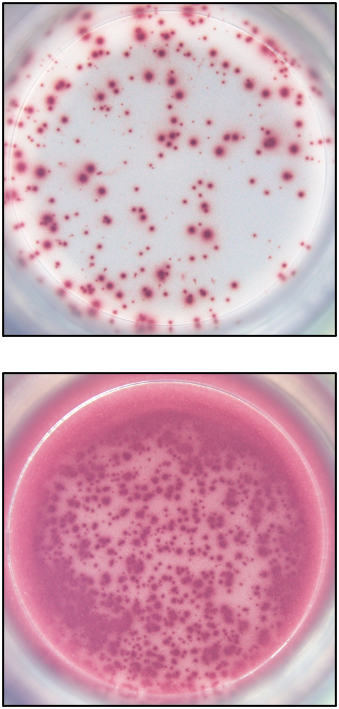
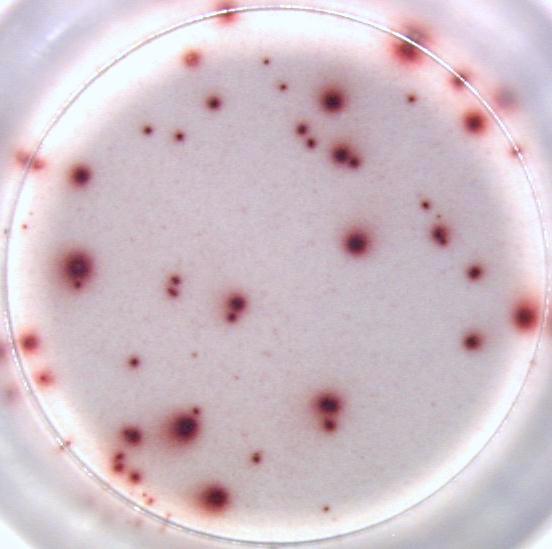
Our ELISPOT kits make use of an enzyme, horseradish peroxidase (HRP), for visualization of the protein secreted by the cells. Picture:
|
|
|
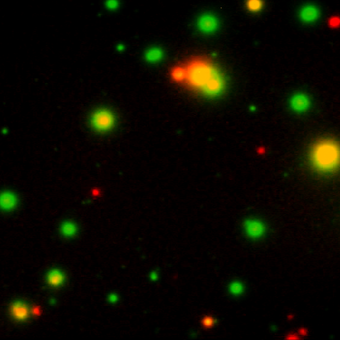
Our FluoroSpot kits use a fluorescent labeled conjugate containing Alexa Fluor 488-labeled anti-FITC antibodies and R-PE-labeled streptavidin. A spot enhancer is used to enhance the fluorescence signal. Picture:
|
 |
| What are the small, dark spots that are not generated by secreted cytokines or antibodies? | |
|
Dust particles can be present in the wells. Upon visual evaluation, these 'spots' vary in size, are highly irregular and usually black. By blowing 4-5 bar compressed air into the well, this problem can easily be solved. See also 'What are the characteristics of a spot?'.
|
|
| What type of plate should I use? | |
|
Our ELISPOT kits are suited for the use with polyvinylidene fluoride (PVDF) membrane-bottomed plates. 96-well MultiScreenHTS Immobilon-P (IP) plates from Millipore (e.g. cat. no. MSIP S4510) are recommended. Our FluoroSpot kits are provided with PVDF membrane-bottomed plates (96-well IP-FL plates from Millipore). The PVDF membrane of these plates is smoother than the plates suitable for ELISPOT assays, resulting in less auto-fluorescence. It is not recommended to use plates suitable for ELISPOT analysis in the FluoroSpot assay. When using hydrophobic PVDF membrane-bottomed plates a prewetting step with 70% ethanol is required. The treatment with ethanol makes the membrane hydrophilic and ensures optimal binding of the coating antibody resulting in better sensitivity (increased spot number) and more accurate quantitation (more sharply defined spots) (Weiss A.J. 2012). After maximum 1 minute of prewetting with 25 µl 70% ethanol, the membrane should be rinsed thoroughly by adding coating buffer to the well to efficiently wash out the ethanol before the coating antibody is added to the wells. Once the membrane is ethanol-treated, it must be kept wet during the entire assay procedure. Overtreatment with large volumes of ethanol, more concentrated ethanol and longer exposure time can lead to trapping of residual liquid between the membrane and underdrain, which may result in poor assay performance. There are both sterile (Millipore cat. no. MSIP S4510 and MSIP S4W10) and non-sterile (Millipore cat. no. MSIP N4510, MSIP N4550, MSIP N4W50 and M8IP S4510) microtiter plates available for ELISPOT analyses. Since ELISPOT requires short culture times and includes the use of antibiotics in the culture medium, non-sterile plates can be safely used without contamination problems. Using clear MultiScreenHTS IP plates (Millipore cat. no. MSIP S4510, MSIP N4510, MSIP N4550 and M8IP S4510) or white MultiScreenHTS IP plates (Millipore cat. no. MSIP S4W10 and MSIP N4W50) with a PVDF membrane bottom is whatever the user prefers best. Both type of plates will give a different appearance in the automated ELISPOT reader. This should not affect the evaluation of the plates. However, the automated ELISPOT reader may find it more difficult to locate the center of the well of white plates due to reflections of the wall. Our 2-plate T cell ELISPOT kits are supplied with 2 PVDF membrane-bottomed plate from Millipore (cat. no. MSIP S4510). We advice to remove the underdrain of the plate after the detection antibody incubation step and to wash both sides of the PVDF membrane in each wash step.
|
|
| What about the washing procedure? | |
|
A critical issue in ELISPOT/FluoroSpot analyses, is the washing procedure. Washing of ELISPOT/FluoroSpot plates is necessary to remove cells and residual biotinylated antibody and staining reagents. We advice to remove the underdrain of the plate after the detection antibody incubation step and to wash both sides of the PVDF membrane in each wash step thereafter. See also 'Direction for washing ELISPOT and FluoroSpot plates'.
|
|
| My question is not mentioned here, how can I contact U-CyTech? | |
|
The researchers of U-CyTech build on >20 years experience in the development and performance of ELISPOT assays for human, monkey, mouse and rat species. They are more than happy to help you with additional advice and to share their experiences. Please contract our customer service: U-CyTech biosciences Phone: +31.85 073 1460 |
|